5 kinds of targeted therapies for the treatment of Mantle Cell Lymphoma

About Mantle Cell Lymphoma (MCL)
Mantle cell lymphoma is a rare type of non-Hodgkin lymphoma that arises in B cells, a type of white blood cell. Most people with mantle cell lymphoma are diagnosed with aggressive, widespread disease. Unlike some other types of aggressive lymphomas, however, mantle cell lymphoma is rarely curable with current therapies.
5 kinds of targeted therapies for the treatment of Mantle Cell Lymphoma can be made in Laos
- Ibrutinib
- Zanubrutinib
- Acalabrutinib
- Orelabrutinib
- Lenalidomide
Targeted therapy
Two Bruton tyrosine kinase inhibitors (BTKi), one In November 2013, ibrutinib (trade name Imbruvica, Pharmacyclics LLC) and in October 2017, acalabrutinib (trade name Calquence, AstraZeneca Pharmaceuticals LP) were approved in the United States for treating MCL. Other targeted agents include the proteasome inhibitor bortezomib, mTOR inhibitors such as temsirolimus, and the P110δ inhibitor GS-1101.
Bruton’s tyrosine kinase (BTK) inhibitors:
A substance that blocks the action of enzymes called tyrosine kinases. Tyrosine kinases are a part of many cell functions, including cell signaling, growth, and division. These enzymes may be too active or found at high levels in some types of cancer cells, and blocking them may help keep cancer cells from growing. Some tyrosine kinase inhibitors are used to treat cancer. They are a type of targeted therapy.
1, Ibrutinib
A drug used alone or with other drugs to treat adults with chronic lymphocytic leukemia, small lymphocytic lymphoma, Waldenstrom macroglobulinemia (a type of non-Hodgkin lymphoma), mantle cell lymphoma, or marginal zone lymphoma. It is also used to treat adults and children aged 1 year and older with chronic graft versus host disease. It is also being studied in the treatment of other types of cancer. Ibrutinib blocks a protein called BTK, which may help keep cancer cells from growing. It may also lower the body’s immune response.
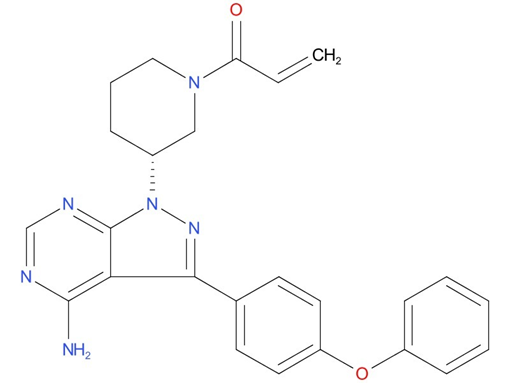
| Drug Profile | Ibrutinib is a small-molecule inhibitor of BTK. Ibrutinib forms a covalent bond with a cysteine residue in the BTK active site, leading to inhibition of BTK enzymatic activity. BTK is a signaling molecule of the B-cell antigen receptor (BCR) and cytokine receptor pathways. BTK’s role in signaling through the B-cell surface receptors results in activation of pathways necessary for B-cell trafficking, chemotaxis, and adhesion. Nonclinical studies show that ibrutinib inhibits malignant B-cell proliferation and survival in vivo as well as cell migration and substrate adhesion in vitro. |
| Alternative Names | CRA-032765; IMBRUVICA; Imbruvica; ImBurvica; JNJ-54179060; PCI-32765 |
| Originator | Celera Genomics Group |
| Developer | Bristol-Myers Squibb; Celgene Corporation; Foundation GIMEMA; Genentech; Janssen; Janssen Biotech; Lymphoma Academic Research Organisation; National Cancer Institute (USA); Northwestern University; OHSU Knight Cancer Institute; Pharmacyclics; Sheba Medical Center; Singapore General Hospital; Stanford University Medical Center; Thomas Jefferson University; University Hospital Muenster; University of California, Davis; University of California, San Diego |
| Class | 2 ring heterocyclic compounds; Antiallergics; Antineoplastics; Antirheumatics; Phenyl ethers; Piperidines; Pyrazoles; Pyrimidines; Small molecules |
| Mechanism of Action | Agammaglobulinaemia tyrosine kinase inhibitors; Emt protein-tyrosine kinase inhibitors |
| Orphan Drug Status | Yes – Chronic lymphocytic leukaemia; Mantle-cell lymphoma; Graft-versus-host disease |
| Patent Information | There are forty patents protecting this compound and three Paragraph IV challenges. Ibrutinib has three hundred and thirty-four patent family members in forty-three countries. |
2, Zanubrutinib
Zanubrutinib is indicated for the treatment of adults with mantle cell lymphoma (MCL) who have received at least one prior therapy, and for the treatment of Waldenström’s macroglobulinemia. It is also indicated for the treatment of adults with relapsed or refractory marginal zone lymphoma (MZL) who have received at least one anti-CD20-based regimen.
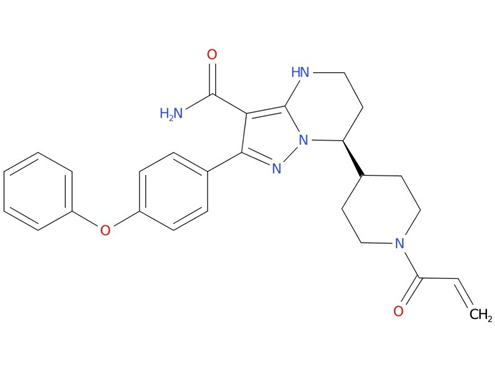
| Drug Profile | zanubrutinib is a Bruton’s tyrosine kinase (BTK) inhibitor. Zanubrutinib forms a covalent bond with a cysteine residue in the BTK active site, leading to inhibition of BTK activity. BTK is a signaling molecule of the B-cell antigen receptor (BCR) and cytokine receptor pathways. In B-cells, BTK signaling results in activation of pathways necessary for B-cell proliferation, trafficking, chemotaxis, and adhesion. In nonclinical studies, zanubrutinib inhibited malignant B-cell proliferation and reduced tumor growth. |
| Alternative Names | BGB-3111; BRUKINSA |
| Originator | BeiGene |
| Developer | BeiGene; Medison Pharma |
| Class | Amides; Antineoplastics; Phenyl ethers; Piperidines; Pyrazoles; Pyrimidines; Small molecules; Urologics |
| Mechanism of Action | Agammaglobulinaemia tyrosine kinase inhibitors |
| Orphan Drug Status | Yes – Waldenstrom’s macroglobulinaemia; Mantle-cell lymphoma; Chronic lymphocytic leukaemia |
| Patent Information | There are four patents protecting this compound. Zanubrutinib has forty-six patent family members in twenty-six countries. |
3, Acalabrutinib
A drug used to treat adults with chronic lymphocytic leukemia or small lymphocytic lymphoma and adults with mantle cell lymphoma that has already been treated with at least one other therapy. It is also being studied in the treatment of other types of cancer. Acalabrutinib blocks a protein called BTK, which may help keep cancer cells from growing. It is a type of tyrosine kinase inhibitor. Also called Calquence.
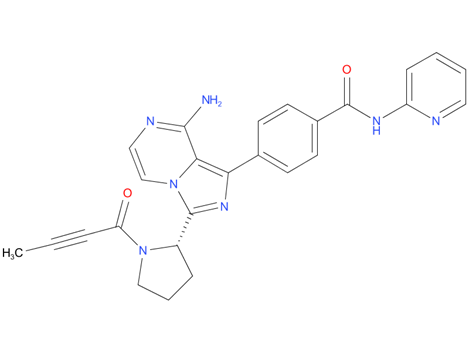
| Drug Profile | Acalabrutinib is a small-molecule inhibitor of BTK. Acalabrutinib and its active metabolite, ACP-5862, form a covalent bond with a cysteine residue in the BTK active site, leading to inhibition of BTK enzymatic activity. BTK is a signaling molecule of the B cell antigen receptor (BCR) and cytokine receptor pathways. In B cells, BTK signaling results in activation of pathways necessary for B-cell proliferation, trafficking, chemotaxis, an adhesion. In nonclinical studies, acalabrutinib inhibited BTK mediated activation of downstream signaling proteins CD86 and CD69 and inhibited malignant B-cell proliferation and survival. |
| Alternative Names | Acalabrutinib maleate; ACP-196; CALQUENCE |
| Originator | Acerta Pharma |
| Developer | Acerta Pharma; AstraZeneca; Biologics Inc; National Institutes of Health (USA) |
| Class | Anti-infectives; Antineoplastics; Antirheumatics; Benzamides; Heavy metals; Imidazoles; Pyrazines; Pyridines; Pyrrolidines; Small molecules |
| Mechanism of Action | Agammaglobulinaemia tyrosine kinase inhibitors |
| Orphan Drug Status | Yes – Mantle-cell lymphoma; Waldenstrom’s macroglobulinaemia; Chronic lymphocytic leukaemia |
| Patent Information | There are seven patents protecting this compound. Acalabrutinib has one hundred and fifty-five patent family members in forty-seven countries. |
4, Orelabrutinib
A small molecule inhibitor of Bruton’s tyrosine kinase (BTK; Bruton agammaglobulinemia tyrosine kinase) with potential antineoplastic activity. Upon administration, orelabrutinib binds to and inhibits the activity of BTK. This prevents both the activation of the B-cell antigen receptor (BCR) signaling pathway and BTK-mediated activation of downstream survival pathways, inhibiting the growth of malignant B cells that overexpress BTK. BTK, a member of the Src-related BTK/Tec family of cytoplasmic tyrosine kinases, is overexpressed or mutated in B-cell malignancies; it plays an important role in the development, activation, signaling, proliferation and survival of B lymphocytes.
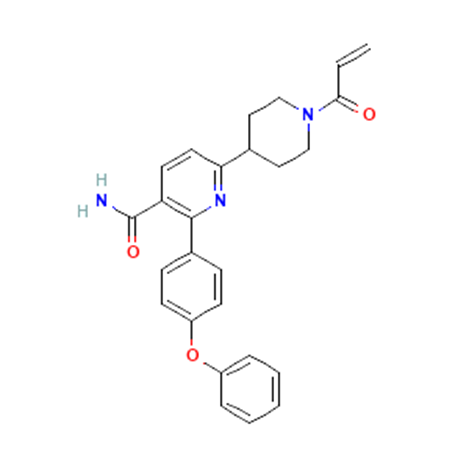
| Drug Profile | Orelabrutinib is a small molecule inhibitor of Bruton’s tyrosine kinase (BTK; Bruton agammaglobulinemia tyrosine kinase) with potential antineoplastic activity. Upon administration, orelabrutinib binds to and inhibits the activity of BTK. This prevents both the activation of the B-cell antigen receptor (BCR) signaling pathway and BTK-mediated activation of downstream survival pathways, inhibiting the growth of malignant B-cells that overexpress BTK. BTK, a member of the Src-related BTK/Tec family of cytoplasmic tyrosine kinases, is overexpressed or mutated in B-cell malignancies; it plays an important role in the development, activation, signaling, proliferation and survival of B-lymphocytes. |
| Alternative Names | BIIB-135; ICP 022; INNOBRUKA; YINUOKAI |
| Originator | InnoCare Pharma |
| Developer | |
| Class | Amides; Anti-inflammatories; Antineoplastics; Antirheumatics; Phenyl ethers; Piperidines; Pyridines; Small molecules |
| Mechanism of Action | Agammaglobulinaemia tyrosine kinase inhibitors |
| Orphan Drug Status | Yes – Mantle-cell lymphoma |
| Patent Information | Null |
5, Lenalidomide
A drug that is similar to thalidomide and is used alone or with other drugs to treat adults with certain types of follicular lymphoma, marginal zone lymphoma, mantle cell lymphoma, multiple myeloma, or anemia caused by certain types of myelodysplastic syndromes. It is also being studied in the treatment of other conditions and types of cancer. Lenalidomide may help the immune system kill abnormal blood cells or cancer cells. It may also prevent the growth of new blood vessels that tumors need to grow. Lenalidomide is a type of antiangiogenesis agent and a type of immunomodulating agent. Also called CC-5013 and Revlimid.
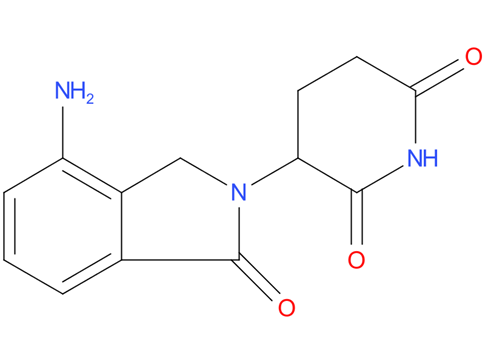
| Drug Profile | Lenalidomide is an analogue of thalidomide with immunomodulatory, antiangiogenic, and antineoplastic properties. Lenalidomide inhibits proliferation and induces apoptosis of certain hematopoietic tumor cells including multiple myeloma, mantle cell lymphoma, and del (5q) myelodysplastic syndromes in vitro. Lenalidomide causes a delay in tumor growth in some in vivo nonclinical hematopoietic tumor models including multiple myeloma. Immunomodulatory properties of lenalidomide include activation of T cells and natural killer (NK) cells, increased numbers of NKT cells, and inhibition of pro-inflammatory cytokines (e.g., TNF-alpha and IL-6) by monocytes. In multiple myeloma cells, the combination of lenalidomide and dexamethasone synergizes the inhibition of cell proliferation and the induction of apoptosis. |
| Alternative Names | CC-5013; CDC-501; CDC-5013; ENMD-0997; IMiD-1; IMiD-3; Ladevina; Revimid™; REVLIMID |
| Originator | Celgene Corporation |
| Developer | Baylor College of Medicine; BeiGene; Celgene Corporation; Dana-Farber Cancer Institute; Groupe dEtude des Lymphomes de lAdulte; H. Lee Moffitt Cancer Center and Research Institute; Helsinki University Central Hospital; Indiana University School of Medicine; IRCCS San Raffaele; Massachusetts General Hospital; National Cancer Institute (USA); Nordic MDS Study Group; Novartis; Ohio State University Comprehensive Cancer Center; Peking Union Medical College Hospital; Roche; Roswell Park Cancer Institute; Royal Marsden NHS Foundation Trust; SCRI Development Innovations; Sidney Kimmel Comprehensive Cancer Center at Johns Hopkins; University of California, Davis; University of Florida; University of Wuerzburg; Washington University School of Medicine; Weill Cornell Medical College |
| Class | 2 ring heterocyclic compounds; Antineoplastics; Imides; Isoindoles; Piperidones; Small molecules |
| Mechanism of Action | Angiogenesis inhibitors; Apoptosis stimulants; Cell proliferation inhibitors; Immunomodulators; Interleukin 6 inhibitors; Tumour necrosis factor alpha inhibitors; Vascular endothelial growth factors inhibitors |
| Orphan Drug Status | Yes – Multiple myeloma; Adult T-cell leukaemia-lymphoma; Myelodysplastic syndromes; Chronic lymphocytic leukaemia |
| Patent Information | There are fifteen patents protecting this compound and three Paragraph IV challenges. Lenalidomide has three hundred and seventy-seven patent family members in forty-one countries. |
Contact us to help you access the Lao pharmaceutical industry
RxLibra started its entrepreneurial journey with the vision of advancing the Lao pharmaceutical industry and becoming a global company. RxLibra is the first company in Laos to focus on exporting life-saving cancer drugs to Asia, Africa and Latin America.
Click & Contact us
The WTO’s Council for Trade-Related Aspects of Intellectual Property Rights (TRIPS) :Under this license, the Lao pharmaceutical industry, as well as the pharmaceutical industry in similar countries(Bangladesh, Nepal, etc.), will be able to manufacture many drugs without patent authorization.
Reference:
《WTO members agree to extend drug patent exemption for poorest members》https://www.wto.org/english/news_e/news15_e/trip_06nov15_e.htm
《Product Patent Protection, the TRIPS LDC Exemption and the Bangladesh Pharmaceutical Industry》https://www.twn.my/title2/IPR/pdf/ipr17.pdf