6 kinds of targeted therapies for the treatment of Acute Lymphocytic Leukemia
About Acute Lymphocytic Leukemia(ALL)
A type of leukemia (blood cancer) that comes on quickly and is fast growing. In acute lymphoblastic leukemia, there are too many lymphoblasts (immature white blood cells) in the blood and bone marrow. Also called acute lymphocytic leukemia and ALL.
6 kinds of targeted drugs for the treatment of Acute Lymphocytic Leukemia can be made in Laos
- Imatinib
- Dasatinib
- Nilotinib
- Ponatinib
- Bosutinib
- Pemigatinib
Targeted therapy
Targeted therapy is a type of treatment that uses drugs or other substances to identify and attack specific cancer cells. Targeted therapies usually cause less harm to normal cells than chemotherapy or radiation therapy do.
Tyrosine kinase inhibitor therapy
This treatment blocks the enzyme, tyrosine kinase, that causes stem cells to develop into more white blood cells (blasts) than the body needs.
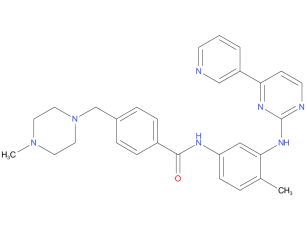
1、Imatinib
Imatinib is used to treat chronic myelogenous leukemia (CML), gastrointestinal stromal tumors (GISTs) and a number of other malignancies. In 2006 the FDA expended approved use to include dermatofibrosarcoma protuberans (DFSP), myelodysplastic/myeloproliferative diseases (MDS/MPD), and aggressive systemic mastocytosis (ASM).
| Drug Profile | A tyrosine kinase inhibitor and ANTINEOPLASTIC AGENT that inhibits the BCR-ABL kinase created by chromosome rearrangements in CHRONIC MYELOID LEUKEMIA and ACUTE LYMPHOBLASTIC LEUKEMIA, as well as PDG-derived tyrosine kinases that are overexpressed in gastrointestinal stromal tumors. |
| Alternative Names | Imatinib Mesylate – Exvastat; Impentri® |
| Originator | Exvastat |
| Developer | Null |
| Class | Amines; Anti-inflammatories; Antineoplastics; Antivirals; Benzamides; Eye disorder therapies; Piperazines; Pyridines; Pyrimidines; Small molecules |
| Mechanism of Action | Angiogenesis inhibitors; Bcr-abl tyrosine kinase inhibitors; Platelet-derived growth factor receptor antagonists; Proto oncogene protein c-kit inhibitors; Signal transduction pathway inhibitors |
| Orphan Drug Status | Null |
| Patent Information | There is one patent protecting this compound and two Paragraph IV challenges,Imatinib mesylate has thirty-five patent family members in twenty-five countries. |
2、Dasatinib
A drug used alone or with other drugs to treat adults and children aged 1 year and older with certain types of chronic myeloid leukemia or acute lymphoblastic leukemia that are Philadelphia chromosome positive. It is also being studied in the treatment of other types of cancer. Dasatinib blocks BCR-ABL and other proteins, which may help keep cancer cells from growing. It is a type of tyrosine kinase inhibitor.
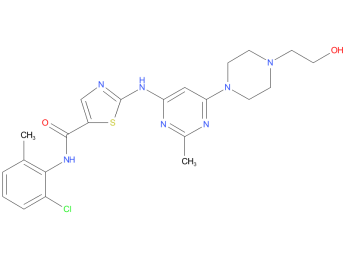
| Drug Profile | A pyrimidine and thiazole derived ANTINEOPLASTIC AGENT and PROTEIN KINASE INHIBITOR of BCR-ABL KINASE. It is used in the treatment of patients with CHRONIC MYELOID LEUKEMIA who are resistant or intolerant to IMATINIB. |
| Alternative Names | BMS-354825; Sprycel |
| Originator | Bristol-Myers Squibb |
| Developer | Bristol-Myers Squibb; City of Hope National Medical Center; Dana-Farber Cancer Institute; Massachusetts General Hospital; Otsuka Pharmaceutical; Sarcoma Alliance for Research through Collaboration; University of Texas M. D. Anderson Cancer Center; Weill Cornell Medical College |
| Class | Amides; Antineoplastics; Chlorobenzenes; Piperazines; Pyrimidines; Small molecules; Thiazoles |
| Mechanism of Action | Bcr-abl tyrosine kinase inhibitors; EphA2 receptor antagonists; Platelet-derived growth factor beta receptor antagonists; Proto oncogene protein c-kit inhibitors; Src-Family kinase inhibitors |
| Orphan Drug Status | Yes – Chronic myeloid leukaemia; Precursor cell lymphoblastic leukaemia-lymphoma |
| Patent Information | There are two patents protecting this compound and three Paragraph IV challenges, Dasatinib has forty-seven patent family members in twenty-nine countries. |
3、Nilotinib
A drug used to treat certain types of chronic myelogenous leukemia that are Philadelphia chromosome positive. It is also being studied in the treatment of other types of cancer. Nilotinib hydrochloride monohydrate blocks BCR-ABL and other proteins, which may help keep cancer cells from growing. It is a type of tyrosine kinase inhibitor.
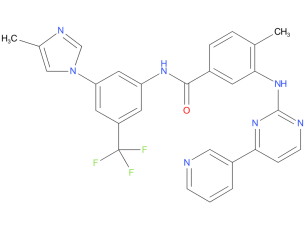
| Drug Profile | Nilotinib is an inhibitor of the BCR-ABL kinase. Nilotinib binds to and stabilizes the inactive conformation of the kinase domain of ABL protein. In vitro, nilotinib inhibited BCR-ABL mediated proliferation of murine leukemic cell lines and human cell lines derived from patients with Ph+ CML. Under the conditions of the assays, nilotinib was able to overcome imatinib resistance resulting from BCR-ABL kinase mutations, in 32 out of 33 mutations tested. In vivo, nilotinib reduced the tumor size in a murine BCR-ABL xenograft model. |
| Alternative Names | AMN-107; Nilotinib-nanoparticle-formulation-Xspray-Pharma; RightSize-nilotinib; Tasigna |
| Originator | Novartis Pharmaceuticals Corporation |
| Developer | Centre Hospitalier Univeristaire De Lille; Dana-Farber Cancer Institute; Hospital for Special Surgery; Novartis Pharmaceuticals Corporation; Seoul St. Mary’s Hospital; University Health Network of Toronto; University of California, San Diego |
| Class | Antineoplastics; Benzamides; Fluorinated hydrocarbons; Imidazoles; Ketones; Pyridines; Pyrimidines; Small molecules |
| Mechanism of Action | Bcr-abl tyrosine kinase inhibitors; Platelet derived growth factor alpha receptor antagonists; Platelet-derived growth factor beta receptor antagonists; Proto oncogene protein c-kit inhibitors |
| Orphan Drug Status | Yes – Chronic myeloid leukaemia; Gastrointestinal stromal tumours; Precursor cell lymphoblastic leukaemia-lymphoma |
| Patent Information | There are seven patents protecting this compound and one Paragraph IV challenge, Nilotinib hydrochloride has two hundred and seventy-six patent family members in fifty-one countries. |
4、Ponatinib
A drug used to treat adults with certain types of chronic myelogenous leukemia or Philadelphia chromosome-positive acute lymphoblastic leukemia. It is used in patients whose cancer has the T315I mutation or whose cancer cannot be treated with other tyrosine kinase inhibitors. It is also being studied in the treatment of other types of cancer. Ponatinib hydrochloride blocks BCR-ABL and other proteins, which may help keep cancer cells from growing and may kill them. It may also prevent the growth of new blood vessels that tumors need to grow. Ponatinib hydrochloride is a type of tyrosine kinase inhibitor and a type of antiangiogenesis agent.
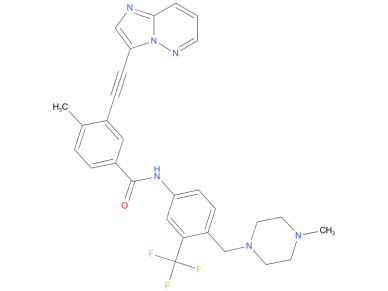
| Drug Profile | a pan-Bcr-Abl protein kinase Inhibitor |
| Alternative Names | AP-24534; AP24534 HCl; AP24534 hydrochloride; AP24534-HCL; Iclusig; Ponatinib hydrochloride |
| Originator | ARIAD Pharmaceuticals |
| Developer | Dana-Farber Cancer Institute; Fundacion CRIS de Investigacion para Vencer el Cancer; Incyte Corporation; Massachusetts General Hospital; Mayo Clinic; National Cancer Institute (USA); Otsuka Beijing Research Institute; Otsuka Pharmaceutical; Takeda; University of Birmingham; University of Colorado at Denver |
| Class | Antineoplastics; Benzamides; Imidazoles; Piperazines; Pyridazines; Small molecules |
| Mechanism of Action | Bcr-abl tyrosine kinase inhibitors; Fibroblast growth factor receptor antagonists; Fms-like tyrosine kinase 3 inhibitors; Platelet derived growth factor alpha receptor antagonists; Proto oncogene protein c ret inhibitors; Proto oncogene protein c-kit inhibitors; Vascular endothelial growth factor receptor antagonis |
| Orphan Drug Status | Yes – Precursor cell lymphoblastic leukaemia-lymphoma; Chronic myeloid leukaemia |
| Patent Information | There are five patents protecting this compound. Ponatinib hydrochloride has ninety-one patent family members in twenty-three countries. |
5、Bosutinib
Bosutinib received US FDA and EU European Medicines Agency approval in September 2012, and March 2013, respectively for the treatment of adults with Philadelphia chromosome-positive (Ph+) chronic myelogenous leukemia (CML) with resistance, or intolerance to prior therapy.
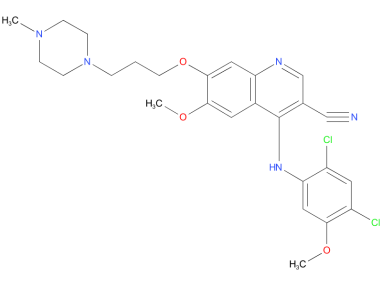
| Drug Profile | a 4-anilino-3-quinolinecarbonitrile dual inhibitor of Src and Abl kinases |
| Alternative Names | Bosulif; PF-05208763; PF-5208763; SKI-606 |
| Originator | Wyeth |
| Developer | Avillion; Massachusetts General Hospital; Pfizer; University of Texas M. D. Anderson Cancer Center |
| Class | Aniline compounds; Antineoplastics; Nitriles; Piperazines; Quinolines; Small molecules; Urologics |
| Mechanism of Action | Bcr-abl tyrosine kinase inhibitors; Src-Family kinase inhibitors |
| Orphan Drug Status | Yes – Chronic myeloid leukaemia |
| Patent Information | There are five patents protecting this compound and two Paragraph IV challenges.Bosutinib monohydrate has eighty-three patent family members in thirty-one countries. |
FGFR2 inhibitors
A patient with an MLN with FGFR1 rearrangement may present with bone marrow involvement with a chronic myeloid malignancy (such as myeloproliferative neoplasm [MPN], myelodysplastic syndrome/MPN) or a blast phase malignancy (such as B- or T-cell acute lymphoblastic leukemia/lymphoma, acute myeloid leukemia or mixed phenotype acute leukemia). Bone marrow involvement may or may not be accompanied by extramedullary disease (EMD);
6、Pemigatinib
Pemigatinib, a fibroblast growth factor receptor (FGFR) inhibitor, is the first targeted treatment approved for use in the United States for treatment of adults with relapsed or refractory myeloid/lymphoid neoplasms (MLNs) with FGFR1 rearrangement.
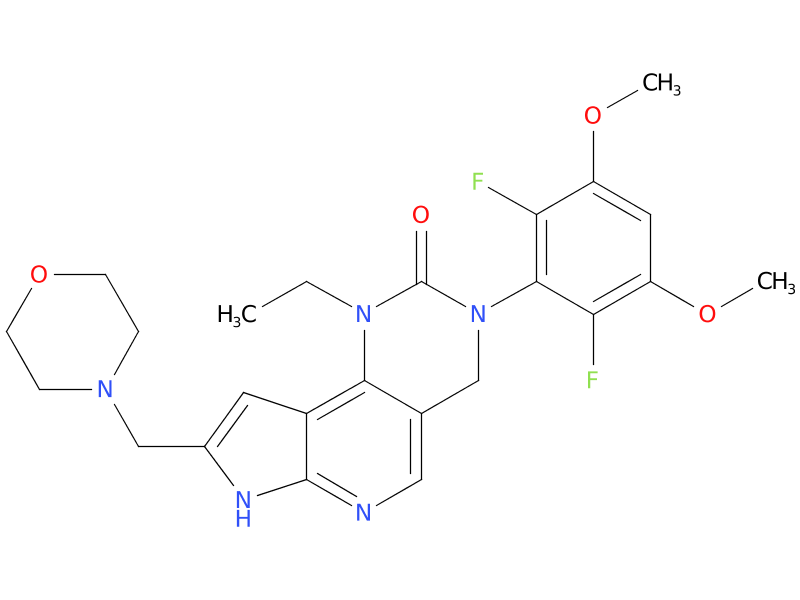
| Drug Profile | Pemigatinib inhibited FGFR1-3 phosphorylation and signaling and decreased cell viability in cancer cell lines with activating FGFR amplifications and fusions that resulted in constitutive activation of FGFR signaling. |
| Alternative Names | IBI-375; INCB 54828; INCB-054828; Pemazyre |
| Originator | Incyte Corporation |
| Developer | Academic and Community Cancer Research United; Incyte Biosciences International; Incyte Corporation; Innovent Biologics; National Cancer Institute (USA) |
| Class | Antineoplastics; Ethers; Fluorobenzenes; Morpholines; Pyridines; Pyrimidinones; Pyrroles; Small molecules |
| Mechanism of Action | Type 1 fibroblast growth factor receptor antagonists; Type 3 fibroblast growth factor receptor antagonists; Type 4 fibroblast growth factor receptor antagonists; Type-2 fibroblast growth factor receptor antagonists |
| Orphan Drug Status | Yes – Cholangiocarcinoma; Myeloproliferative disorders; Lymphoma |
| Patent Information | This drug has ninety-five patent family members in thirty-seven countries.Pemazyre will be eligible for patent challenges on April 17, 2024. |
Contact us to help you access the Lao pharmaceutical industry
RxLibra started its entrepreneurial journey with the vision of advancing the Lao pharmaceutical industry and becoming a global company. RxLibra is the first company in Laos to focus on exporting life-saving cancer drugs to Asia, Africa and Latin America.
Click & Contact us ?
The WTO’s Council for Trade-Related Aspects of Intellectual Property Rights (TRIPS) :Under this license, the Lao pharmaceutical industry, as well as the pharmaceutical industry in similar countries(Bangladesh, Nepal, etc.), will be able to manufacture many drugs without patent authorization.
Reference:
《WTO members agree to extend drug patent exemption for poorest members》https://www.wto.org/english/news_e/news15_e/trip_06nov15_e.htm
《Product Patent Protection,the TRIPS LDC Exemption and the Bangladesh Pharmaceutical Industry》https://www.twn.my/title2/IPR/pdf/ipr17.pdf