4 kinds of targeted therapies for the treatment of Follicular Lymphoma

About Follicular Lymphoma(FL)
Follicular lymphoma (FL) is a cancer that involves certain types of white blood cells known as lymphocytes. The cancer originates from the uncontrolled division of specific types of B-cells known as centrocytes and centroblasts. These cells normally occupy the follicles (nodular swirls of various types of lymphocytes) in the germinal centers of lymphoid tissues such as lymph nodes. The cancerous cells in FL typically form follicular or follicle-like structures (see adjacent Figure) in the tissues they invade. These structures are usually the dominant histological feature of this cancer.
4 kinds of targeted drugs for the treatment of Follicular Lymphoma can be made in Laos
- Tazemetostat
- Copanlisib
- Duvelisib
- Umbralisib
EZH2 inhibitor
EZH2, which belongs to the class of histone methyltransferases (HMTs), is overexpressed or mutated in a variety of cancer cells and plays a key role in tumor cell proliferation.
1.Tazemetostat
Tazemetostat is an orally available, small molecule selective and S-adenosyl methionine (SAM) competitive inhibitor of histone methyl transferase EZH2, with potential antineoplastic activity. Upon oral administration, tazemetostat selectively inhibits the activity of both wild-type and mutated forms of EZH2. Inhibition of EZH2 specifically prevents the methylation of histone H3 lysine 27 (H3K27). This decrease in histone methylation alters gene expression patterns associated with cancer pathways and results in decreased tumor cell proliferation in EZH2 mutated cancer cells.
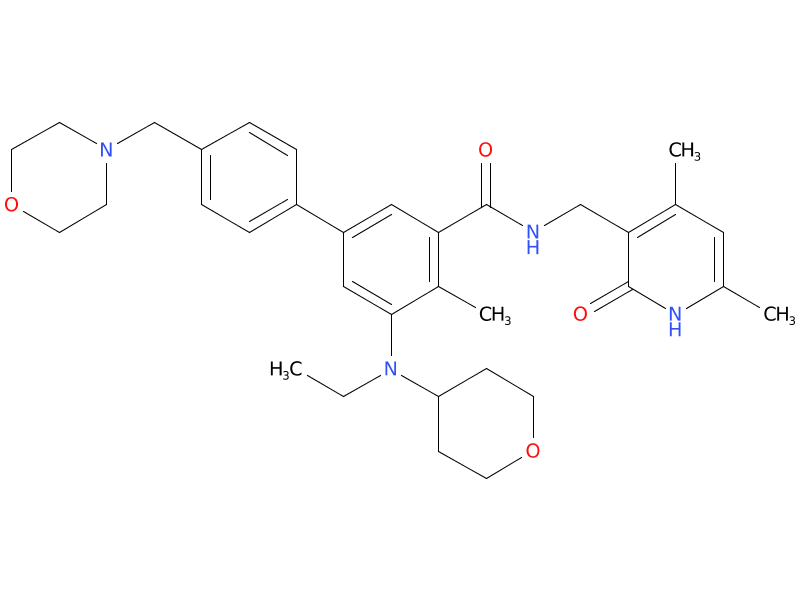
| Drug Profile | Tazemetostat is an inhibitor of the methyltransferase, EZH2, and some EZH2 gain-of-function mutations including Y646X and A687V. Tazemetostat also inhibited EZH1 with a half-maximal inhibitory concentration (IC50) of 392 nM, approximately 36 times higher than the IC50 for inhibition of EZH2. |
| Alternative Names | Davico; E-7438; EPZ-6438; EZ-438; Tazemetostat hydrobromide; Tazestat – Epizyme; Tazverik; Zestastat – Epizyme |
| Originator | Epizyme |
| Developer | Eisai Co Ltd; Epizyme; Genentech; HUTCHMED; National Cancer Institute (USA); Pfizer |
| Class | Amides; Amines; Antineoplastics; Biphenyl compounds; Dihydropyridines; Morpholines; Small molecules |
| Mechanism of Action | Enhancer of zeste homolog 2 protein inhibitors; SMARCA2 protein inhibitors; SMARCA4 protein inhibitors |
| Orphan Drug Status | Yes – Chordoma; Follicular lymphoma; Soft tissue sarcoma; Diffuse large B cell lymphoma; Rhabdoid tumour; Mesothelioma |
| Patent Information | There are twenty-three patents protecting this compound. Tazemetostat hydrobromide has three hundred and twenty-four patent family members in thirty-eight countries. |
PI3K inhibitors
Phosphoinositide 3-kinase inhibitors (PI3K inhibitors) are a class of medical drugs that are mainly used to treat advanced cancers. They function by inhibiting one or more of the phosphoinositide 3-kinase (PI3K) enzymes, which are part of the PI3K/AKT/mTOR pathway. This signal pathway regulates cellular functions such as growth and survival. It is strictly regulated in healthy cells, but is always active in many cancer cells, allowing the cancer cells to better survive and multiply. PI3K inhibitors block the PI3K/AKT/mTOR pathway and thus slow down cancer growth. They are examples of a targeted therapy.
2. Copanlisib
mainly targets the PI3K-alpha and PI3K-delta proteins. This drug can be used to treat follicular lymphoma, typically after other treatments have been tried. It’s given as an infusion into a vein, typically once a week for 3 weeks, followed by a week off.
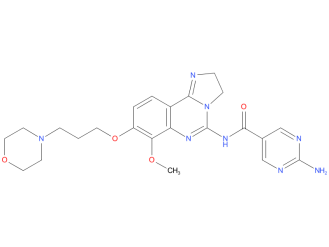
| Drug Profile | Copanlisib is an inhibitor of phosphatidylinositol-3-kinase (PI3K) with inhibitory activity predominantly against PI3K-alpha and PI3K-delta isoforms expressed in malignant B cells. Copanlisib has been shown to induce tumor cell death by apoptosis and inhibition of proliferation of primary malignant B cell lines. Copanlisib inhibits several key cell-signaling pathways, including B-cell receptor (BCR) signaling, CXCR12 mediated chemotaxis of malignant B cells, and NFkB signaling in lymphoma cell lines. |
| Alternative Names | 5-Pyrimidinecarboxamide,2-Amino-N-(2,3-dihydro-7-methoxy-8-(3-(4-morpholinyl) propoxy)imidazo(1,2-C)quinazolin-5-yl)-, Hydrochloride (1:2)Aliqopa; Aliqopa; BAY-80-6946 Dihydrochloride; BAY-806946; BAY-841236; Copanlisib Dihydrochloride; Copanlisib hydrochloride |
| Originator | Bayer Schering Pharma; Memorial Sloan-Kettering Cancer Center; National Cancer Institute (USA) |
| Developer | All Ireland Cooperative Oncology Research Group; Bayer HealthCare Pharmaceuticals; Bristol-Myers Squibb; Chonnam National University Hospital; H. Lee Moffitt Cancer Center and Research Institute; Memorial Sloan-Kettering Cancer Center; National Cancer Institute (USA); Sidney Kimmel Comprehensive Cancer Center at Johns Hopkins; UNICANCER; University Hospital Muenster; University of Washington |
| Class | 3-ring heterocyclic compounds; Amides; Antineoplastics; Imidazoles; Morpholines; Pyrimidines; Quinazolines; Small molecules |
| Mechanism of Action | Phosphatidylinositol 3 kinase alpha inhibitors; Phosphatidylinositol 3 kinase delta inhibitors |
| Orphan Drug Status | Yes – Follicular lymphoma; Marginal zone B-cell lymphoma; Chronic lymphocytic leukaemia; Waldenstrom’s macroglobulinaemia |
| Patent Information | There are four patents protecting this compound. Copanlisib dihydrochloride has one hundred and fifty-three patent family members in fifty countries. |
3.Duvelisib
Duvelisib is a PI3K inhibitor that blocks two kinase proteins called PI3K-delta and PI3K-gamma, the FDA approved Duvelisib for the treatment of a Adult patients with relapsed or refractory follicular lymphoma (FL) after at least two prior systemic therapies.
| Drug Profile | Duvelisib is an inhibitor of PI3K with inhibitory activity predominantly against PI3K-delta and PI3K-gamma isoforms expressed in normal and malignant B-cells. Duvelisib induced growth inhibition and reduced viability in cell lines derived from malignant B-cells and in primary CLL tumor cells. Duvelisib inhibits several key cell-signaling pathways, including B-cell receptor signaling and CXCR12-mediated chemotaxis of malignant B-cells. Additionally, duvelisib inhibits CXCL12-induced T cell migration and M-CSF and IL-4 driven M2 polarization of macrophages. |
| Alternative Names | ABBV-954; COPIKTRA; INK-1197; IPI-145; VS-0145; YHI-1702 |
| Originator | Intellikine |
| Developer | AbbVie; CSPC Pharmaceutical Group; Dana-Farber Cancer Institute; Infinity Pharmaceuticals; Memorial Sloan-Kettering Cancer Center; Secura Bio; Verastem Oncology; Yakult Honsha |
| Class | Anti-inflammatories; Antiasthmatics; Antineoplastics; Antirheumatics; Isoquinolines; Purines; Small molecules |
| Mechanism of Action | Phosphatidylinositol 3 kinase delta inhibitors; Phosphatidylinositol 3 kinase gamma inhibitors |
| Orphan Drug Status | Yes – T-cell lymphoma; Chronic lymphocytic leukaemia; Peripheral T-cell lymphoma; Follicular lymphoma |
| Patent Information | There are five patents protecting this compound. This drug has one hundred and forty-seven patent family members in thirty-four countries. |
4.Umbralisib
Umbralisib was indicated for adults with relapsed or refractory marginal zone lymphoma (MZL) who have received at least one prior anti-CD20-based regimen; and adults with relapsed or refractory follicular lymphoma (FL) who have received at least three prior lines of systemic therapy.
| Drug Profile | Umbralisib inhibits multiple kinases. In biochemical and cell-based assays, umbralisib inhibited PI3Kdelta and casein kinase CK1-epsilon. PI3K-delta is expressed in normal and malignant B-cells; CK1-epsilon has been implicated in the pathogenesis of cancer cells, including lymphoid malignancies. Umbralisib also inhibited a mutated form of ABL1 in biochemical assays. Umbralisib inhibited cell proliferation, CXCL12-mediated cell adhesion, and CCL19-mediated cell migration in lymphoma cell lines in studies conducted in vitro. |
| Alternative Names | RP 5307; RP-5264; TGR-1202; UKONIQ |
| Originator | Incozen Therapeutics; Rhizen Pharmaceuticals |
| Developer | TG Therapeutics Inc |
| Class | Amines; Antineoplastics; Benzopyrans; Fluorinated hydrocarbons; Ketones; Pyrazoles; Pyrimidines; Small molecules |
| Mechanism of Action | Casein kinase Iepsilon inhibitors; Phosphatidylinositol 3 kinase delta inhibitors |
| Orphan Drug Status | Yes – Follicular lymphoma; Diffuse large B cell lymphoma; Chronic lymphocytic leukaemia; Marginal zone B-cell lymphoma |
| Patent Information | There are eight patents protecting this compound. Umbralisib tosylate has sixty-eight patent family members in thirty-one countries. |
Contact us to help you access the Lao pharmaceutical industry
RxLibra started its entrepreneurial journey with the vision of advancing the Lao pharmaceutical industry and becoming a global company. RxLibra is the first company in Laos to focus on exporting life-saving cancer drugs to Asia, Africa and Latin America.
Click & Contact us?
The WTO’s Council for Trade-Related Aspects of Intellectual Property Rights (TRIPS) :Under this license, the Lao pharmaceutical industry, as well as the pharmaceutical industry in similar countries(Bangladesh, Nepal, etc.), will be able to manufacture many drugs without patent authorization.
Reference:
《WTO members agree to extend drug patent exemption for poorest members》https://www.wto.org/english/news_e/news15_e/trip_06nov15_e.htm
《Product Patent Protection,the TRIPS LDC Exemption and the Bangladesh Pharmaceutical Industry》https://www.twn.my/title2/IPR/pdf/ipr17.pdf