1 kind of targeted therapies for the treatment of Mucosa-Associated Lymphoid Tissue

About Mucosa-Associated Lymphoid Tissue (MALT)
The mucosa-associated lymphoid tissue (MALT), also called mucosa-associated lymphatic tissue, is a diffuse system of small concentrations of lymphoid tissue found in various submucosal membrane sites of the body, such as the gastrointestinal tract, nasopharynx, thyroid, breast, lung, salivary glands, eye, and skin. MALT is populated by lymphocytes such as T cells and B cells, as well as plasma cells and macrophages, each of which is well situated to encounter antigens passing through the mucosal epithelium. In the case of intestinal MALT, M cells are also present, which sample antigen from the lumen and deliver it to the lymphoid tissue. MALT constitute about 50% of the lymphoid tissue in human body. Immune responses that occur at mucous membranes are studied by mucosal immunology.
1 kind of targeted therapies for the treatment of Mucosa-Associated Lymphoid Tissue can be made in Laos
- Ibrutinib
Targeted therapy
MALT lymphoma in the stomach doesn’t respond to antibiotics if the lymphoma is not linked with H. pylori infection or if the lymphoma comes back (recurs) after treatment. The targeted therapy drug rituximab may be given alone or combined with chemotherapy.
Bruton’s tyrosine kinase (BTK) inhibitors:
Bruton’s tyrosine kinase (BTK) inhibitors work by binding to the BTK protein. BTK inhibitors block this protein’s activity by the BCR-induced BTK activation and its downstream signalling. BTK inhibitors block the activity that leads to growth of the B-cells and this causes cell death of the malignant B-cells.
1, Ibrutinib
A drug used alone or with other drugs to treat adults with chronic lymphocytic leukemia, small lymphocytic lymphoma, Waldenstrom macroglobulinemia (a type of non-Hodgkin lymphoma), mantle cell lymphoma, or marginal zone lymphoma. It is also used to treat adults and children aged 1 year and older with chronic graft versus host disease. It is also being studied in the treatment of other types of cancer. Ibrutinib blocks a protein called BTK, which may help keep cancer cells from growing. It may also lower the body’s immune response.
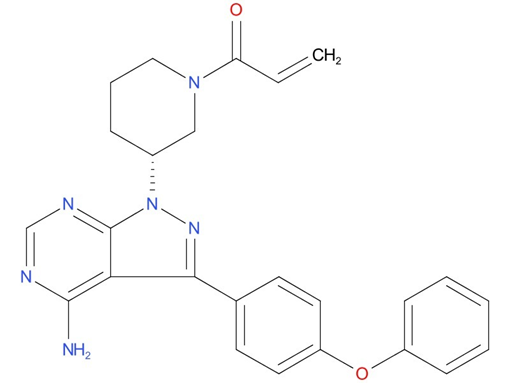
| Drug Profile | Ibrutinib is a small-molecule inhibitor of BTK. Ibrutinib forms a covalent bond with a cysteine residue in the BTK active site, leading to inhibition of BTK enzymatic activity. BTK is a signaling molecule of the B-cell antigen receptor (BCR) and cytokine receptor pathways. BTK’s role in signaling through the B-cell surface receptors results in activation of pathways necessary for B-cell trafficking, chemotaxis, and adhesion. Nonclinical studies show that ibrutinib inhibits malignant B-cell proliferation and survival in vivo as well as cell migration and substrate adhesion in vitro. |
| Alternative Names | CRA-032765; IMBRUVICA; Imbruvica; ImBurvica; JNJ-54179060; PCI-32765 |
| Originator | Celera Genomics Group |
| Developer | Bristol-Myers Squibb; Celgene Corporation; Foundation GIMEMA; Genentech; Janssen; Janssen Biotech; Lymphoma Academic Research Organisation; National Cancer Institute (USA); Northwestern University; OHSU Knight Cancer Institute; Pharmacyclics; Sheba Medical Center; Singapore General Hospital; Stanford University Medical Center; Thomas Jefferson University; University Hospital Muenster; University of California, Davis; University of California, San Diego |
| Class | 2 ring heterocyclic compounds; Antiallergics; Antineoplastics; Antirheumatics; Phenyl ethers; Piperidines; Pyrazoles; Pyrimidines; Small molecules |
| Mechanism of Action | Agammaglobulinaemia tyrosine kinase inhibitors; Emt protein-tyrosine kinase inhibitors |
| Orphan Drug Status | Yes – Chronic lymphocytic leukaemia; Mantle-cell lymphoma; Graft-versus-host disease |
| Patent Information | There are forty patents protecting this compound and three Paragraph IV challenges.Ibrutinib has three hundred and thirty-four patent family members in forty-three countries. |
Contact us to help you access the Lao pharmaceutical industry
RxLibra started its entrepreneurial journey with the vision of advancing the Lao pharmaceutical industry and becoming a global company. RxLibra is the first company in Laos to focus on exporting life-saving cancer drugs to Asia, Africa and Latin America.
Click & Contact us
The WTO’s Council for Trade-Related Aspects of Intellectual Property Rights (TRIPS) :Under this license, the Lao pharmaceutical industry, as well as the pharmaceutical industry in similar countries(Bangladesh, Nepal, etc.), will be able to manufacture many drugs without patent authorization.
Reference:
《WTO members agree to extend drug patent exemption for poorest members》https://www.wto.org/english/news_e/news15_e/trip_06nov15_e.htm
《Product Patent Protection, the TRIPS LDC Exemption and the Bangladesh Pharmaceutical Industry》https://www.twn.my/title2/IPR/pdf/ipr17.pdf