About Non Small Cell Lung Carcinoma (NSCLC)
Non-small-cell lung carcinoma (NSCLC) is any type of epithelial lung cancer other than small-cell lung carcinoma (SCLC). NSCLC accounts for about 85% of all lung cancers. As a class, NSCLCs are relatively insensitive to chemotherapy, compared to small-cell carcinoma. When possible, they are primarily treated by surgical resection with curative intent, although chemotherapy has been used increasingly both preoperatively (neoadjuvant chemotherapy) and postoperatively (adjuvant chemotherapy).
5 kinds of targeted drugs for the treatment of About ALK-positive non-small cell lung cancer can be made in Laos
- Crizotinib
- Ceritinib
- Alectinib
- Brigatinib
- Lorlatinib
Targeted therapy
Targeted therapy is a type of treatment that uses drugs or other substances to identify and attack specific cancer cells. Targeted therapies usually cause less harm to normal cells than chemotherapy or radiation therapy do. Monoclonal antibodies, tyrosine kinase inhibitors, and mammalian target of rapamycin (mTOR) inhibitors are three types of targeted therapy being used to treat advanced, metastatic, or recurrent non-small cell lung cancer.
ALK Inhibitors:
A gene that makes a protein that is involved in cell growth. Mutated (changed) forms of the ALK gene and protein have been found in some types of cancer, including neuroblastoma, non-small cell lung cancer, and anaplastic large cell lymphoma. These changes may increase the growth of cancer cells. Checking for changes in the ALK gene in tumor tissue may help to plan cancer treatment. Also called anaplastic lymphoma kinase gene.
1, Crizotinib
Crizotinib is indicated for the treatment of metastatic non-small cell lung cancer (NSCLC) or relapsed or refractory, systemic anaplastic large cell lymphoma (ALCL) that is ALK-positive. It is also indicated for the treatment of unresectable, recurrent, or refractory inflammatory anaplastic lymphoma kinase (ALK)-positive myofibroblastic tumors (IMT).
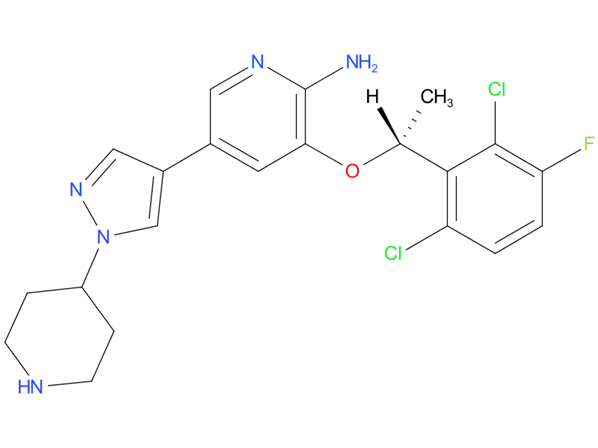
| Drug Profile | Crizotinib is an inhibitor of receptor tyrosine kinases including ALK, Hepatocyte Growth Factor Receptor (HGFR, c-Met), ROS1 (c-ros), and Recepteur d’Origine Nantais (RON). Translocations can affect the ALK gene resulting in the expression of oncogenic fusion proteins. The formation of ALK fusion proteins results in activation and dysregulation of the gene’s expression and signaling which can contribute to increased cell proliferation and survival in tumors expressing these proteins. Crizotinib demonstrated concentration-dependent inhibition of ALK, ROS1, and c-Met phosphorylation in cell-based assays using tumor cell lines and demonstrated antitumor activity in mice bearing tumor xenografts that expressed echinoderm microtubule-associated protein-like 4 (EML4)- or nucleophosmin (NPM)-ALK fusion proteins or c-Met. |
| Alternative Names | PF-002341066; PF-02341066; PF-1066; PF-2341066; Xalkori |
| Originator | Pfizer |
| Developer | Astellas Pharma; Children’s Oncology Group; Dana-Farber Cancer Institute; National Cancer Institute (USA); OxOnc Development; Pfizer; University of Colorado at Denver; University of Milan Bicocca |
| Class | Antineoplastics; Chlorobenzenes; Fluorobenzenes; Piperidines; Pyrazoles; Pyridines; Small molecules |
| Mechanism of Action | Anaplastic lymphoma kinase inhibitors; Proto oncogene protein c met inhibitors; ROS1 protein inhibitors |
| Orphan Drug Status | Yes – Neuroblastoma; Non-small cell lung cancer; Non-Hodgkin’s lymphoma |
| Patent Information | There are five patents protecting this compound. Crizotinib has one hundred and fifty patent family members in forty-seven countries. |
2, Ceritinib
A drug used to treat adults with non-small cell lung cancer that has spread and is ALK positive. It is also being studied in the treatment of other types of cancer. Ceritinib blocks certain proteins made by the ALK gene. Blocking these proteins may stop the growth and spread of cancer cells. Ceritinib is a type of tyrosine kinase inhibitor. Also called Zykadia.
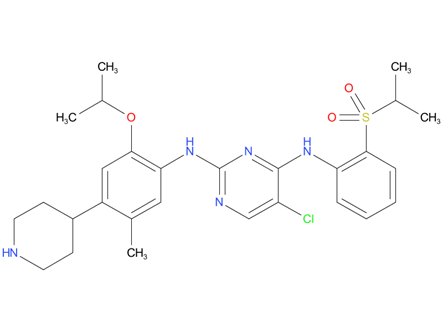
| Drug Profile | Ceritinib is a kinase inhibitor. Targets of ceritinib inhibition identified in either biochemical or cellular assays at clinically relevant concentrations include ALK, insulin-like growth factor 1 receptor (IGF-1R), insulin receptor (InsR), and ROS1. Among these, ceritinib is most active against ALK. Ceritinib inhibited autophosphorylation of ALK, ALK-mediated phosphorylation of the downstream signaling protein STAT3, and proliferation of ALK-dependent cancer cells in in vitro and in vivo assays. |
| Alternative Names | Jikadia; LDK-378; NVP-LDK 378; NVP-LDK378-NX; Zykadia |
| Originator | Novartis |
| Developer | H. Lee Moffitt Cancer Center and Research Institute; Novartis; University of Texas Southwestern Medical Center |
| Class | Antineoplastics; Diamines; Piperidines; Pyrimidines; Small molecules; Sulfones |
| Mechanism of Action | Anaplastic lymphoma kinase inhibitors |
| Orphan Drug Status | Yes – Non-small cell lung cancer |
| Patent Information | There are eleven patents protecting this compound. Ceritinib has three hundred and eighty-five patent family members in fifty-five countries. |
3, Alectinib
A drug used to treat non-small cell lung cancer that has spread and is ALK positive. It is also being studied in the treatment of other types of cancer. Alectinib blocks certain proteins made by the ALK gene. Blocking these proteins may stop the growth and spread of cancer cells.
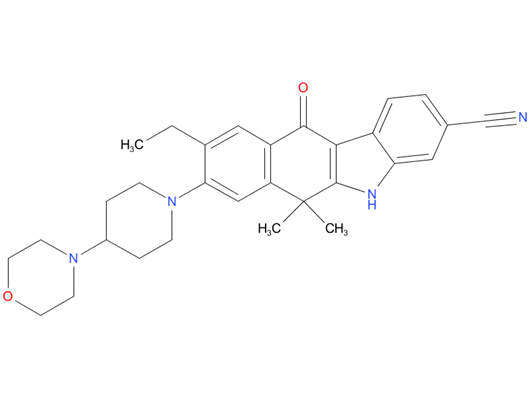
| Drug Profile | Alectinib is a tyrosine kinase inhibitor that targets ALK and RET. In nonclinical studies, alectinib inhibited ALK phosphorylation and ALK-mediated activation of the downstream signaling proteins STAT3 and AKT, and decreased tumor cell viability in multiple cell lines harboring ALK fusions, amplifications, or activating mutations. The major active metabolite of alectinib, M4, showed similar in vitro potency and activity. |
| Alternative Names | AF-802; Alecensa; ALECENSARO; Alectinib hydrochloride; CH-5424802; RG-7853; RO-5424802; RO-5452802 |
| Originator | Chugai Pharmaceutical |
| Developer | Chugai Pharmaceutical; National Hospital Organization Nagoya Medical Center; Roche; Seoul National University Hospital; University College London |
| Class | Antineoplastics; Carbazoles; Morpholines; Nitriles; Phenyl ethers; Piperidines; Small molecules |
| Mechanism of Action | Anaplastic lymphoma kinase inhibitors |
| Orphan Drug Status | Yes – Non-small cell lung cancer; Anaplastic large cell lymphoma |
| Patent Information | There are four patents protecting this compound. Alectinib hydrochloride has one hundred and twenty-three patent family members in thirty-eight countries. |
4, brigatinib
A drug used to treat adults with non-small cell lung cancer that has spread and is ALK positive. It is also being studied in the treatment of other types of cancer. Brigatinib blocks certain proteins made by the ALK gene. Blocking these proteins may stop the growth and spread of cancer cells. Brigatinib is a type of tyrosine kinase inhibitor. Also called Alunbrig.
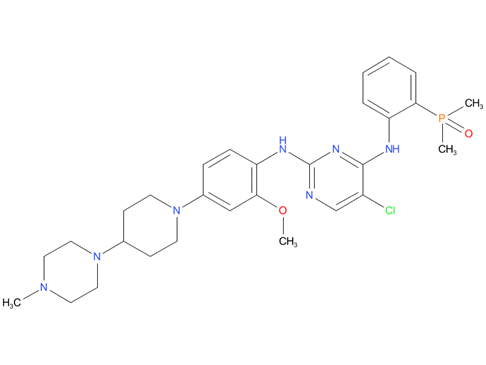
| Drug Profile | Brigatinib is a tyrosine kinase inhibitor with in vitro activity at clinically achievable concentrations against multiple kinases including ALK, ROS1, insulin-like growth factor-1 receptor (IGF-1R), and FLT-3 as well as EGFR deletion and point mutations. Brigatinib inhibited autophosphorylation of ALK and ALK-mediated phosphorylation of the downstream signaling proteins STAT3, AKT, ERK1/2, and S6 in in vitro and in vivo assays. Brigatinib also inhibited the in vitro proliferation of cell lines expressing EML4-ALK and NPM-ALK fusion proteins and demonstrated dose-dependent inhibition of EML4-ALK-positive NSCLC xenograft growth in mice. At clinically achievable concentrations (<= 500 nM), brigatinib inhibited the in vitro viability of cells expressing EML4-ALK and 17 mutant forms associated with resistance to ALK inhibitors including crizotinib, as well as EGFR-Del (E746-A750), ROS1-L2026M, FLT3-F691L, and FLT3-D835Y. Brigatinib exhibited in vivo anti-tumor activity against 4 mutant forms of EML4-ALK, including G1202R and L1196M mutants identified in NSCLC tumors in patients who have progressed on crizotinib. Brigatinib also reduced tumor burden and prolonged survival in mice implanted intracranially with an ALK-driven tumor cell line. |
| Alternative Names | ALUNBRIG; AP-26113 |
| Originator | ARIAD Pharmaceuticals |
| Developer | Takeda |
| Class | Antineoplastics; Diamines; Piperazines; Piperidines; Pyrimidines; Small molecules |
| Mechanism of Action | Anaplastic lymphoma kinase inhibitors; Cytochrome P-450 enzyme system stimulants; Epidermal growth factor receptor antagonists; ROS1 protein inhibitors |
| Orphan Drug Status | Yes – Non-small cell lung cancer |
| Patent Information | There are four patents protecting this compound. Brigatinib has eighty-six patent family members in thirty-seven countries. |
5, Lorlatinib
A drug used to treat adults with non-small cell lung cancer that has spread to other parts of the body and is ALK positive. It is also being studied in the treatment of other types of cancer. Lorlatinib blocks certain proteins made by the ALK gene. Blocking these proteins may stop the growth and spread of cancer cells.
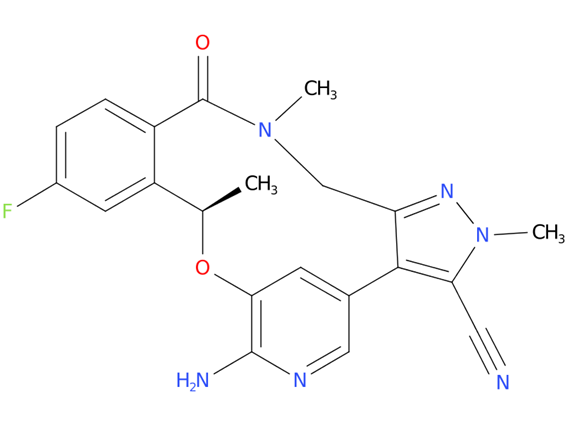
| Drug Profile | Lorlatinib is a kinase inhibitor with in vitro activity against ALK and ROS1 as well as TYK1, FER, FPS, TRKA, TRKB, TRKC, FAK, FAK2, and ACK. Lorlatinib demonstrated in vitro activity against multiple mutant forms of the ALK enzyme, including some mutations detected in tumors at the time of disease progression on crizotinib and other ALK inhibitors. |
| Alternative Names | [14C]Lorlatinib; LORBRENA; LORVIQUA; Lorviqua; PF-06463922; PF-6463922 |
| Originator | Pfizer |
| Developer | CStone Pharmaceuticals; New Approaches to Neuroblastoma Therapy Consortium; Pfizer; The EVAN Foundation; University of Southern California |
| Class | Antineoplastics; Aza compounds; Benzoxazines; Nitriles; Pyrazoles; Pyridines; Small molecules |
| Mechanism of Action | Anaplastic lymphoma kinase inhibitors; ROS1 protein inhibitors |
| Orphan Drug Status | Yes – Non-small cell lung cancer |
| Patent Information | There are four patents protecting this compound. Lorlatinib has ninety-five patent family members in forty-seven countries. |
Contact us to help you access the Lao pharmaceutical industry
RxLibra started its entrepreneurial journey with the vision of advancing the Lao pharmaceutical industry and becoming a global company. RxLibra is the first company in Laos to focus on exporting life-saving cancer drugs to Asia, Africa and Latin America.
Click & Contact us 👇
The WTO’s Council for Trade-Related Aspects of Intellectual Property Rights (TRIPS) :Under this license, the Lao pharmaceutical industry, as well as the pharmaceutical industry in similar countries(Bangladesh, Nepal, etc.), will be able to manufacture many drugs without patent authorization.
Reference:
《WTO members agree to extend drug patent exemption for poorest members》https://www.wto.org/english/news_e/news15_e/trip_06nov15_e.htm
《Product Patent Protection, the TRIPS LDC Exemption and the Bangladesh Pharmaceutical Industry》https://www.twn.my/title2/IPR/pdf/ipr17.pdf
Comments are closed