3 targeted drugs for Bile Duct Cancer
About Bile Duct Cancer (Cholangiocarcinoma)
Bile duct cancer is a rare disease in which malignant (cancer) cells form in the bile ducts. Bile duct cancer is also called cholangiocarcinoma (CCAs).CCAs are highly aggressive tumors that display features of biliary differentiation. CCAs can arise either within the liver, termed intrahepatic cholangiocarcinoma (iCCA), or in the perihilar or distal portions of the draining bile ducts (perihilar or distal CCA, respectively).
Cholangiocarcinoma, or bile duct cancer, It often affects older adults and has usually spread beyond the bile ducts by the time it’s diagnosed. Treatment usually involves a combination of surgery, chemotherapy or radiation therapy.
3 Targeted Therapy Drugs for Cholangiocarcinoma cancer can be ordered in Laos
Targeted therapies target specific parts of cancer cells. Some people with bile duct cancer that’s the result of an abnormal gene have specific proteins on their cancer cells. Targeted therapies can attack these cells to keep them from dividing.Targeted therapies usually cause less harm to normal cells than chemotherapy or radiation therapy do. The following targeted therapies are being studied in patients with bile duct cancer that is locally advanced and cannot be removed by surgery or has spread to other parts of the body:
- Ivosidenib
- Pemigatinib
- Infigratinib
IDH inhibitors
IDH1 is one of three isocitrate dehydrogenase isozymes, the other two being IDH2 and IDH3, and encoded by one of five isocitrate dehydrogenase genes, which are IDH1, IDH2, IDH3A, IDH3B, and IDH3G.IDH1 mutations are heterozygous, typically involving an amino acid substitution in the active site of the enzyme in codon 132. The mutation results in a loss of normal enzymatic function and the abnormal production of 2-hydroxyglutarate (2-HG).It has been considered to take place due to a change in the binding site of the enzyme.2-HG has been found to inhibit enzymatic function of many alpha-ketoglutarate dependent dioxygenases, including histone and DNA demethylases, causing widespread changes in histone and DNA methylation and potentially promoting tumorigenesis.
1、Ivosidenib
Ivosidenib is an IDH1 inhibitor. Ivosidenib is approved to treat adults whose cancer has a certain mutation in the IDH1 gene.
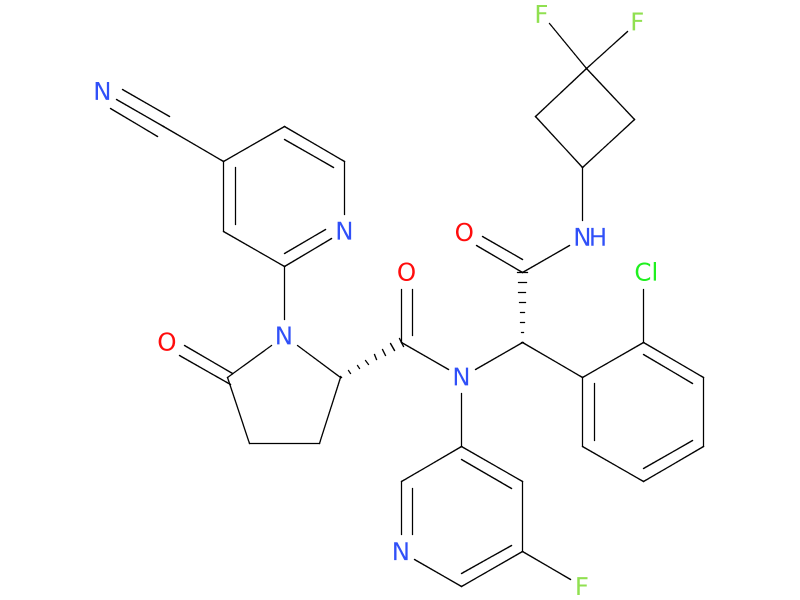
| Drug Profile | Ivosidenib is a small molecule inhibitor that targets the mutant isocitrate dehydrogenase 1 (IDH1) enzyme. Susceptible IDH1 mutations are defined as those leading to increased levels of 2-hydroxyglutarate(2-HG) in the leukemia cells and where efficacy is predicted by 1) clinically meaningful remissions with the recommended dose of ivosidenib and/or 2) inhibition of mutant IDH1 enzymatic activity at concentrations of ivosidenib sustainable at the recommended dosage according to validated methods. The most common of such mutations are R132H and R132C substitutions. |
| Alternative Names | AG-120; CS 3010; S-95031; TIBSOVO |
| Originator | Agios Pharmaceuticals |
| Developer | Agios Pharmaceuticals; Bristol-Myers Squibb; CStone Pharmaceuticals; HOVON Foundation; University of Pittsburgh Medical Center |
| Class | Antineoplastics; Cyclobutanes; Nitriles; Pyridines; Pyrrolidines; Small molecules |
| Mechanism of Action | Isocitrate dehydrogenase 1 inhibitors |
| Orphan Drug Status | Yes – Acute myeloid leukaemia; Cholangiocarcinoma; Glioma |
| Patent Information | This drug has one hundred and fourteen patent family members in thirty-seven countries.Tibsovo was eligible for patent challenges on July 20, 2022. |
FGFR2 inhibitors
One of the most frequent genetic events in patients with iCCAs are fusions that involve the fibroblast growth factor receptor 2 (FGFR2).The portfolio of available FGFR-inhibitory compounds quickly expanded,and the growing interest in FGFR inhibitors as targeted therapy for FGFR2-fusion positive iCCA .
2、Pemigatinib
Pemigatinib is approved to treat Cholangiocarcinoma (bile duct cancer) that has metastasized (spread to other parts of the body) or is locally advanced and cannot be treated by surgery. It is used in adults who have been treated for disease that has a FGFR2 gene fusion or other change in the structure of the FGFR2 gene.
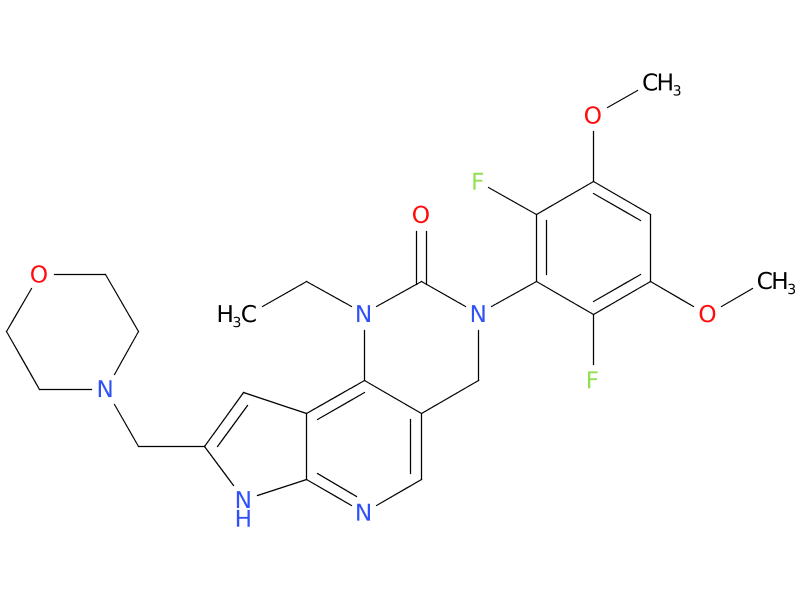
| Drug Profile | Pemigatinib inhibited FGFR1-3 phosphorylation and signaling and decreased cell viability in cancer cell lines with activating FGFR amplifications and fusions that resulted in constitutive activation of FGFR signaling. |
| Alternative Names | IBI-375; INCB 54828; INCB-054828; Pemazyre |
| Originator | Incyte Corporation |
| Developer | Academic and Community Cancer Research United; Incyte Biosciences International; Incyte Corporation; Innovent Biologics; National Cancer Institute (USA) |
| Class | Antineoplastics; Ethers; Fluorobenzenes; Morpholines; Pyridines; Pyrimidinones; Pyrroles; Small molecules |
| Mechanism of Action | Type 1 fibroblast growth factor receptor antagonists; Type 3 fibroblast growth factor receptor antagonists; Type 4 fibroblast growth factor receptor antagonists; Type-2 fibroblast growth factor receptor antagonists |
| Orphan Drug Status | Yes – Cholangiocarcinoma; Myeloproliferative disorders; Lymphoma |
| Patent Information | This drug has ninety-five patent family members in thirty-seven countries.Pemazyre will be eligible for patent challenges on April 17, 2024. |
3、Infigratinib
Infigratinib phosphate is approved to treat Cholangiocarcinoma (bile duct cancer) that has spread and cannot be removed with surgery. It is used in adults whose cancer has been treated and has an FGFR2 gene fusion or other change in the structure of the FGFR2 gene.
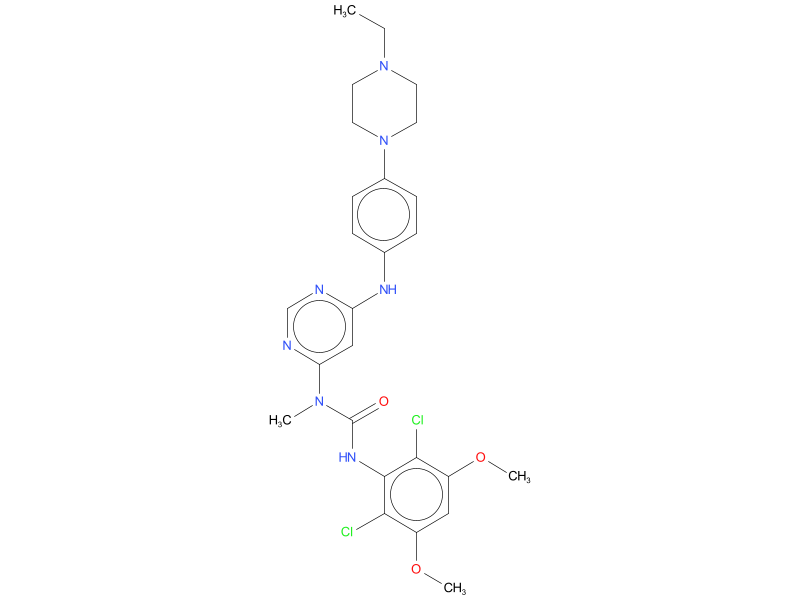
| Drug Profile | Infigratinib is a small molecule kinase inhibitor of FGFR with IC50 values of 1.1, 1, 2, and 61 nM for FGFR1, FGFR2, FGFR3, and FGFR4, respectively. The major human metabolites of infigratinib, BHS697 and CQM157, have similar in vitro binding affinities for FGFR1, FGFR2, and FGFR3 compared to infigratinib. Infigratinib inhibited FGFR signaling and decreased cell proliferation in cancer cell lines with activating FGFR amplifications, mutations, or fusions. Constitutive FGFR signaling can support the proliferation and survival of malignant cells. Infigratinib had anti-tumor activity in mouse and rat xenograft models of human tumors with activating FGFR2 or FGFR3 alterations, including two patient-derived xenograft models of cholangiocarcinoma that expressed FGFR2-TTC28 or FGFR2-TRA2B fusions. Infigratinib demonstrated brain-to-plasma concentration ratios (based on AUC0-inf) of 0.682 in rats after a single oral dose. |
| Alternative Names | BBP-831; BGJ-398; BGJ-398 phosphate; Infigratinib phosphate; NVP-BGJ398; TRUSELTIQ |
| Originator | Novartis |
| Developer | Array BioPharma; Helsinn; LianBio; Novartis; Novartis Oncology; QED Therapeutics |
| Class | Aniline compounds; Antineoplastics; Chlorobenzenes; Methylurea compounds; Phenyl ethers; Piperazines; Pyrimidines; Small molecules |
| Mechanism of Action | Type 1 fibroblast growth factor receptor antagonists; Type 3 fibroblast growth factor receptor antagonists; Type 4 fibroblast growth factor receptor antagonists; Type-2 fibroblast growth factor receptor antagonists |
| Orphan Drug Status | Yes – Cholangiocarcinoma |
| Patent Information | This drug has one hundred and thirty patent family members in forty countries.Truseltiq will be eligible for patent challenges on May 28, 2025. |
Contact us to help you access the Lao pharmaceutical industry
RxLibra started its entrepreneurial journey with the vision of advancing the Lao pharmaceutical industry and becoming a global company. RxLibra is the first company in Laos to focus on exporting life-saving cancer drugs to Asia, Africa and Latin America.
Click & Contact us ?
The WTO’s Council for Trade-Related Aspects of Intellectual Property Rights (TRIPS) :Under this license, the Lao pharmaceutical industry, as well as the pharmaceutical industry in similar countries(Bangladesh, Nepal, etc.), will be able to manufacture many drugs without patent authorization.
Reference:
《WTO members agree to extend drug patent exemption for poorest members》https://www.wto.org/english/news_e/news15_e/trip_06nov15_e.htm
《Product Patent Protection,the TRIPS LDC Exemption and the Bangladesh Pharmaceutical Industry》https://www.twn.my/title2/IPR/pdf/ipr17.pdf