2 drugs for the treatment of Myelodysplastic syndrome
About Myelodysplastic syndromes (MDS)
Myelodysplastic syndromes (MDS) are conditions that can occur when the blood-forming cells in the bone marrow become abnormal. This leads to low numbers of one or more types of blood cells. MDS is considered a type of cancer.In about 1 in 3 patients, MDS can progress to a rapidly growing cancer of bone marrow cells called acute myeloid leukemia (AML).
2 drugs for the Treatment of myelodysplasia can be ordered in Laos
- Lenalidomide
- Eltrombopag
Immunomodulating drugs (IMiDs)
Lenalidomide belongs to a class of drugs known as immunomodulating drugs (IMiDs). It seems to work well in low-grade MDS, often eliminating the need for blood transfusions, at least for a time. The drug seems to work best in people whose MDS cells are missing a part of chromosome 5 (MDS-del(5q)). But it can also help some MDS patients that do not have this abnormal chromosome
1、Lenalidomide
enalidomide is an analogue of thalidomide with immunomodulatory, antiangiogenic, and antineoplastic properties. Lenalidomide inhibits proliferation and induces apoptosis of certain hematopoietic tumor cells including multiple myeloma, mantle cell lymphoma, and del (5q) myelodysplastic syndromes in vitro. Lenalidomide causes a delay in tumor growth in some in vivo nonclinical hematopoietic tumor models including multiple myeloma.
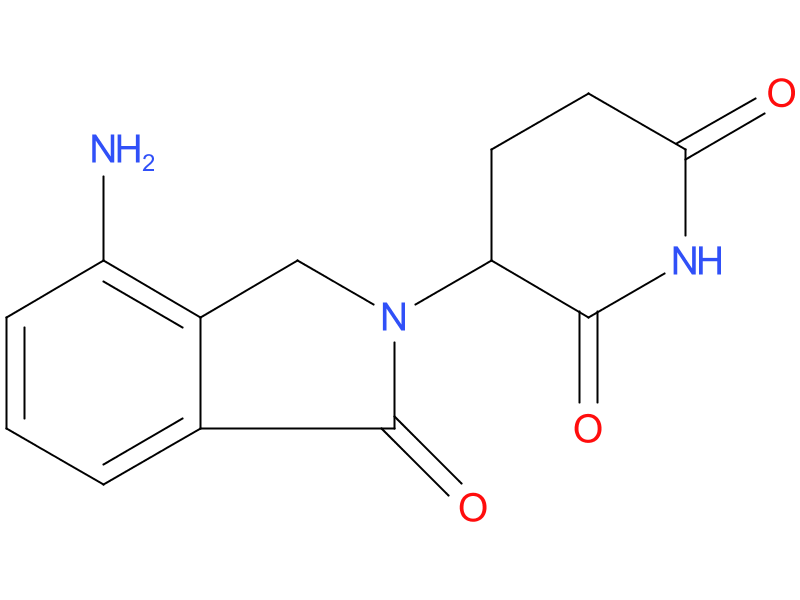
| Drug Profile | Immunomodulatory properties of lenalidomide include activation of T cells and natural killer (NK) cells, increased numbers of NKT cells, and inhibition of pro-inflammatory cytokines (e.g., TNF-alpha and IL-6) by monocytes. |
| Alternative Names | CMIO-LLD; STAR LLD programme; STAR-LLD; STAR-LLD SC |
| Originator | ChemioCare |
| Developer | Starton Therapeutics |
| Class | 2 ring heterocyclic compounds; Antineoplastics; Imides; Isoindoles; Piperidones; Small molecules |
| Mechanism of Action | Janus kinase 1 inhibitors; Janus kinase-2 inhibitors |
| Orphan Drug Status | Angiogenesis inhibitors; Apoptosis stimulants; Cell proliferation inhibitors; Immunomodulators; Interleukin 6 inhibitors; Tumour necrosis factor alpha inhibitors; Vascular endothelial growth factors inhibitors |
| Patent Information | There are fifteen patents protecting this compound and three Paragraph IV challenges. |
Platelet growth factors
Drugs called thrombopoietin-receptor agonists, such as romiplostim and eltrombopag might help some people with MDS who have very low platelet levels.
2、Eltrombopag
Eltrombopag, a thrombopoietin- receptor agonist in the treatment of adult chronic immune thrombocytopenia
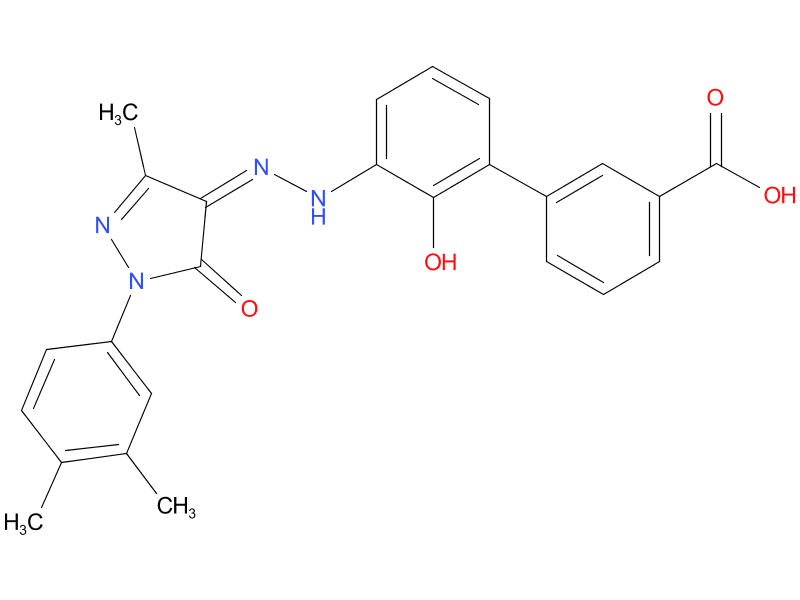
| Drug Profile | an oral, nonpeptide thrombopoietin receptor agonist |
| Alternative Names | 497115; Eltrombopag olamine; ETB-115; Promacta; Reboredo; Revolade; SB-497115; SB-497115-GR |
| Originator | Ligand Pharmaceuticals |
| Developer | French Innovative Leukemia Organisation; GlaxoSmithKline; Groupe Francophone des Myelodysplasies; Novartis; Technische Universitat Dresden |
| Class | Antianaemics; Antineoplastics; Benzoates; Biphenyl compounds; Carboxylic acids; Hydrazines; Pyrazoles; Radioprotectives; Small molecules |
| Mechanism of Action | Thrombopoietin receptor agonists |
| Orphan Drug Status | Yes – Aplastic anaemia; Idiopathic thrombocytopenic purpura |
| Patent Information | Eltrombopag olamine is the generic ingredient in two branded drugs marketed by Novartis and is included in two NDAs. There are eight patents protecting this compound and two Paragraph IV challenges. |
Contact us to help you access the Lao pharmaceutical industry
RxLibra started its entrepreneurial journey with the vision of advancing the Lao pharmaceutical industry and becoming a global company. RxLibra is the first company in Laos to focus on exporting life-saving cancer drugs to Asia, Africa and Latin America.
Click & Contact us?
The WTO’s Council for Trade-Related Aspects of Intellectual Property Rights (TRIPS) :Under this license, the Lao pharmaceutical industry, as well as the pharmaceutical industry in similar countries(Bangladesh, Nepal, etc.), will be able to manufacture many drugs without patent authorization.
Reference:
《WTO members agree to extend drug patent exemption for poorest members》https://www.wto.org/english/news_e/news15_e/trip_06nov15_e.htm
《Product Patent Protection,the TRIPS LDC Exemption and the Bangladesh Pharmaceutical Industry》https://www.twn.my/title2/IPR/pdf/ipr17.pdf