6 kinds of targeted therapies for the treatment of multiple Myeloma

About Multiple Myeloma (MM)
Multiple myeloma cells are abnormal plasma cells (a type of white blood cell) that build up in the bone marrow and form tumors in many bones of the body. Normal plasma cells make antibodies to help the body fight infection and disease. As the number of multiple myeloma cells increases, more antibodies are made. This can cause the blood to thicken and keep the bone marrow from making enough healthy blood cells. Multiple myeloma cells also damage and weaken the bone.
6 kinds of targeted therapies for the treatment of multiple Myeloma can be made in Laos
- Panobinostat
- Thalidomide
- Lenalidomide
- Pomalidomide
- Selinexor
- Ixazomib
Targeted therapy
Targeted therapy is a type of treatment that uses drugs or other substances to identify and attack specific cancer cells. Targeted therapies usually cause less harm to normal cells than chemotherapy or radiation therapy do. Several types of targeted therapy may be used to treat multiple myeloma and other plasma cell neoplasms. There are different types of targeted therapy:
Proteasome inhibitor therapy:
This treatment blocks the action of proteasomes in cancer cells. A proteasome is a protein that removes other proteins no longer needed by the cell. When the proteins are not removed from the cell, they build up and may cause the cancer cell to die.
1, Panobinostat
Panobinostat is used in combination with the anti-cancer drug bortezomib and the corticoid dexamethasone for the treatment of multiple myeloma in adults who had received at least two previous treatments, including bortezomib and an immunomodulatory agent.
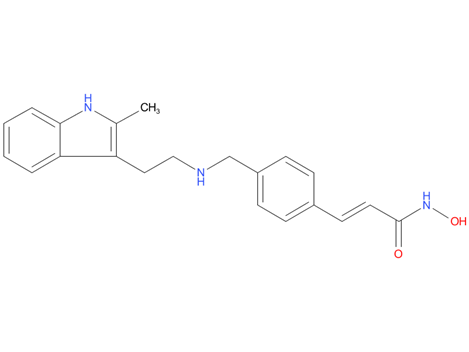
| Drug Profile | An indole and hydroxamic acid derivative that acts as a HISTONE DEACETYLASE inhibitor. It is used as an antineoplastic agent in combination with BORTEZOMIB and DEXAMETHASONE for the treatment of MULTIPLE MYELOMA. |
| Alternative Names | Faridak; Farydak; LBH-589; LBH-589A |
| Originator | Novartis |
| Developer | Georgia Regents University; H. Lee Moffitt Cancer Center and Research Institute; HOVON Foundation; Icahn School of Medicine at Mount Sinai; Mayo Clinic; National Cancer Institute (USA); New York University School of Medicine; Novartis; Peter MacCallum Cancer Centre; Secura Bio; St. Jude Childrens Research Hospital; Therapeutic Advances in Childhood Leukemia & Lymphoma; Thomas Jefferson University; University of Aarhus; University of California at San Francisco; University of Texas M. D. Anderson Cancer Center; University of Wisconsin-Madison |
| Class | Antineoplastics; Hydroxamic acids; Indoles; Small molecules |
| Mechanism of Action | Histone deacetylase inhibitors |
| Orphan Drug Status | Yes – Multiple myeloma |
| Patent Information | There are two patents protecting this compound. Panobinostat lactate has sixty-seven patent family members in forty countries. |
2, Thalidomide
Thalidomide is used as a first-line treatment in multiple myeloma in combination with dexamethasone or with melphalan and prednisone to treat acute episodes of erythema nodosum leprosum and for maintenance therapy.
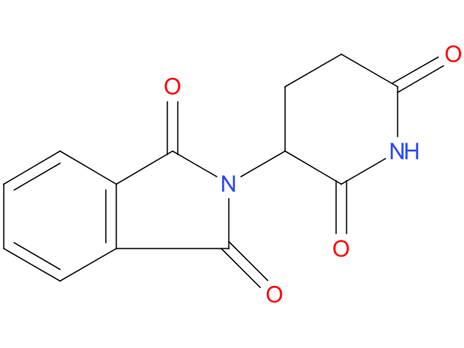
| Drug Profile | A piperidinyl isoindole originally introduced as a non-barbiturate hypnotic, but withdrawn from the market due to teratogenic effects. It has been reintroduced and used for a number of immunological and inflammatory disorders. Thalidomide displays immunosuppressive and anti-angiogenic activity. It inhibits release of TUMOR NECROSIS FACTOR-ALPHA from monocytes, and modulates other cytokine action. |
| Alternative Names | Alpha-N-phthalimidoglutarimide; CC-2001; FPF-300; K-17; NSC-66847; Synovir; Thaled; Thalidomide Celgene; Thalidomide Pharmion; Thalomid |
| Originator | EntreMed; National Cancer Institute (USA); National Institute of Allergy and Infectious Diseases; National Institutes of Health (USA); University of Pennsylvania |
| Developer | Celgene Corporation; Erkim; Fujimoto Pharmaceutical; Laphal; Weill Cornell Medical College |
| Class | Antifibrotics; Antileprotics; Antineoplastics; Indoles; Phthalimides; Piperidones; Small molecules |
| Mechanism of Action | Angiogenesis inhibitors; Immunosuppressants; Tumour necrosis factor inhibitors |
| Orphan Drug Status | Yes – Graft-versus-host disease; Cachexia; Multiple myeloma; Aphthous stomatitis; Leprosy; Crohn’s disease; Kaposi’s sarcoma; POEMS syndrome; Mycobacterial infections; Myelodysplastic syndromes; Brain cancer |
| Patent Information | There is one patent protecting this compound and three Paragraph IV challenges. Thalidomide has twenty-nine patent family members in nineteen countries. |
3, Lenalidomide
Lenalidomide is used to treat multiple myeloma. It is a more potent molecular analog of thalidomide, which inhibits tumor angiogenesis, tumor-secreted cytokines, and tumor proliferation through induction of apoptosis.
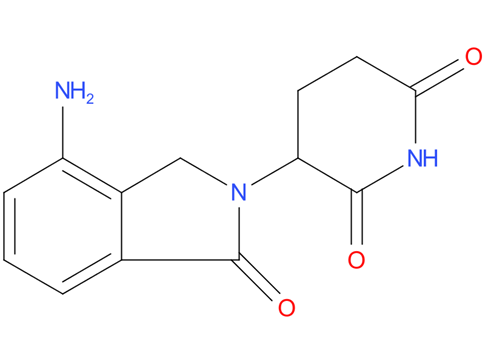
| Drug Profile | Lenalidomide is an analogue of thalidomide with immunomodulatory, antiangiogenic, and antineoplastic properties. Lenalidomide inhibits proliferation and induces apoptosis of certain hematopoietic tumor cells including multiple myeloma, mantle cell lymphoma, and del (5q) myelodysplastic syndromes in vitro. Lenalidomide causes a delay in tumor growth in some in vivo nonclinical hematopoietic tumor models including multiple myeloma. Immunomodulatory properties of lenalidomide include activation of T cells and natural killer (NK) cells, increased numbers of NKT cells, and inhibition of pro-inflammatory cytokines (e.g., TNF-alpha and IL-6) by monocytes. In multiple myeloma cells, the combination of lenalidomide and dexamethasone synergizes the inhibition of cell proliferation and the induction of apoptosis. |
| Alternative Names | CC-5013; CDC-501; CDC-5013; ENMD-0997; IMiD-1; IMiD-3; Ladevina; Revimid™; REVLIMID |
| Originator | Celgene Corporation |
| Developer | Baylor College of Medicine; BeiGene; Celgene Corporation; Dana-Farber Cancer Institute; Groupe dEtude des Lymphomes de lAdulte; H. Lee Moffitt Cancer Center and Research Institute; Helsinki University Central Hospital; Indiana University School of Medicine; IRCCS San Raffaele; Massachusetts General Hospital; National Cancer Institute (USA); Nordic MDS Study Group; Novartis; Ohio State University Comprehensive Cancer Center; Peking Union Medical College Hospital; Roche; Roswell Park Cancer Institute; Royal Marsden NHS Foundation Trust; SCRI Development Innovations; Sidney Kimmel Comprehensive Cancer Center at Johns Hopkins; University of California, Davis; University of Florida; University of Wuerzburg; Washington University School of Medicine; Weill Cornell Medical College |
| Class | 2 ring heterocyclic compounds; Antineoplastics; Imides; Isoindoles; Piperidones; Small molecules |
| Mechanism of Action | Angiogenesis inhibitors; Apoptosis stimulants; Cell proliferation inhibitors; Immunomodulators; Interleukin 6 inhibitors; Tumour necrosis factor alpha inhibitors; Vascular endothelial growth factors inhibitors |
| Orphan Drug Status | Yes – Multiple myeloma; Adult T-cell leukaemia-lymphoma; Myelodysplastic syndromes; Chronic lymphocytic leukaemia |
| Patent Information | There are fifteen patents protecting this compound and three Paragraph IV challenges. Lenalidomide has three hundred and seventy-seven patent family members in forty-one countries. |
4, Pomalidomide
A drug that is similar to thalidomide and is used alone or with other drugs to treat adults with certain types of multiple myeloma or Kaposi sarcoma. It is also being studied in the treatment of other types of cancer. Pomalidomide may help the immune system kill cancer cells. It may also prevent the growth of new blood vessels that tumors need to grow. Pomalidomide is a type of immunomodulating agent and a type of antiangiogenesis agent.
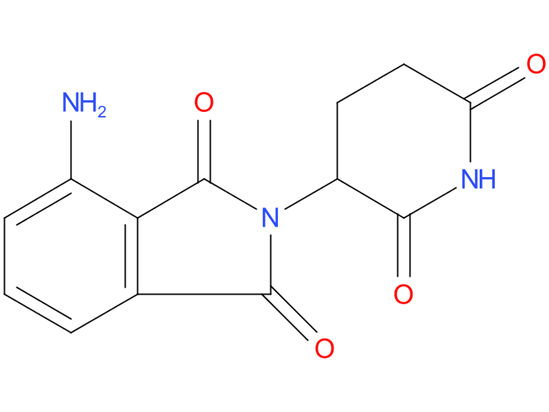
| Drug Profile | an immunomodulator with antineoplastic and angiogenesis inhibitor activity |
| Alternative Names | Actimid; CC-4047; CDC-394; Imnovid; Pomalidomide-Celgene; Pomalyst |
| Originator | Celgene Corporation |
| Developer | Bristol-Myers Squibb; Celgene Corporation; Dana-Farber Cancer Institute; Sarah Cannon Research Institute; University of Texas M. D. Anderson Cancer Center |
| Class | Anti-inflammatories; Antianaemics; Antineoplastics; Phthalimides; Piperidones; Skin disorder therapies; Small molecules |
| Mechanism of Action | Angiogenesis inhibitors; Apoptosis stimulants; Interleukin 6 inhibitors; Natural killer cell stimulants; T lymphocyte stimulants; Tumour necrosis factor alpha inhibitors |
| Orphan Drug Status | Yes – Myelofibrosis; Multiple myeloma; Kaposi’s sarcoma |
| Patent Information | There are six patents protecting this compound and one Paragraph IV challenge. Pomalidomide has three hundred and fifty-six patent family members in forty-eight countries. |
5, selinexor
Selinexor is approved in combination with bortezomib and dexamethasone for the treatment of adults with multiple myeloma who have received at least one prior therapy. Selinexor is also approved for use in combination with the steroid dexamethasone in people with relapsed or refractory multiple myeloma who have received at least four prior therapies and whose disease is refractory to at least two proteosome inhibitors, at least two immunomodulatory agents, and an anti-CD38 monoclonal antibody (so-called “quad-refractory” or “penta-refractory” myeloma), for whom no other treatment options are available. It is the first drug to be approved for this indication.
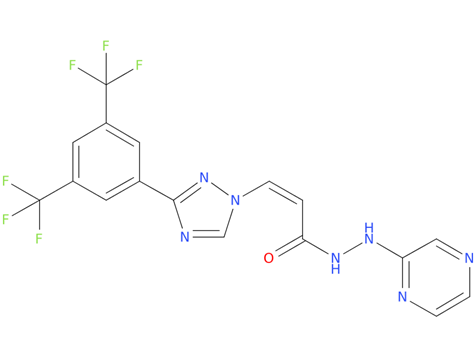
| Drug Profile | Selinexor reversibly inhibits nuclear export of tumor suppressor proteins (TSPs), growth regulators, and mRNAs of oncogenic proteins by blocking exportin 1(XPO1) |
| Alternative Names | ATG-010; CRM1-nuclear-export-inhibitor; KPT-330; NEXPOVIO; ONO 7705; Selinisso; XPOVIO |
| Originator | Karyopharm Therapeutics |
| Developer | Antengene Corporation; Cardiff University; Grupo Espanol de Investigacion en Sarcomas; Karyopharm Therapeutics; Ono Pharmaceutical; Promedico; University of Texas Southwestern Medical Center |
| Class | Acrylamides; Anti-infectives; Antineoplastics; Antivirals; Fluorinated hydrocarbons; Foot disorder therapies; Hydrazines; Pyrazines; Small molecules; Triazoles |
| Mechanism of Action | Exportin-1 protein inhibitors |
| Orphan Drug Status | Yes – Diffuse large B cell lymphoma; Acute myeloid leukaemia; Chronic lymphocytic leukaemia; Multiple myeloma; Myelofibrosis; Soft tissue sarcoma |
| Patent Information | There are six patents protecting this compound. Selinexor has one hundred and seven patent family members in thirty-six countries. |
6.Ixazomib
Ixazomib is used in combination with lenalidomide and dexamethasone for the treatment of multiple myeloma in adults after at least one prior therapy. There are no experiences with children and youths under 18 years of age.
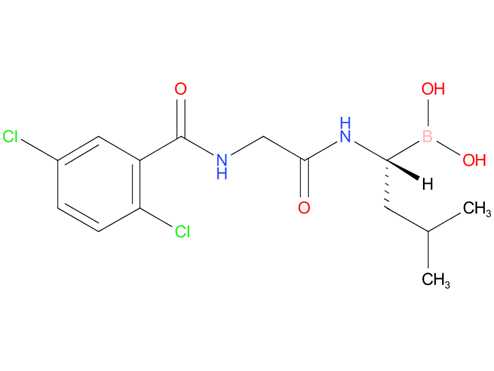
| Drug Profile | an oral proteasome inhibitor with antineoplastic activity; MLN2238 is the biologically active form of MLN9708 |
| Alternative Names | Ixazomib citrate; MLN-2238; MLN-9708; NINLARO |
| Originator | Millennium Pharmaceuticals; University of California, San Diego |
| Developer | Celgene Corporation; Helsinki University Central Hospital; HOVON Foundation; Massachusetts General Hospital; National Cancer Institute (USA); Northside Hospital; Northwestern University; Takeda Oncology; University of Texas M. D. Anderson Cancer Center |
| Class | Amides; Antineoplastics; Antivirals; Chlorobenzenes; Organic boron compounds; Small molecules |
| Mechanism of Action | Apoptosis stimulants; Proteasome inhibitors |
| Orphan Drug Status | Yes – Multiple myeloma; Amyloidosis |
| Patent Information | There are nine patents protecting this compound. Ixazomib citrate has two hundred and twenty-one patent family members in forty-five countries. |
Contact us to help you access the Lao pharmaceutical industry
RxLibra started its entrepreneurial journey with the vision of advancing the Lao pharmaceutical industry and becoming a global company. RxLibra is the first company in Laos to focus on exporting life-saving cancer drugs to Asia, Africa and Latin America.
Click & Contact us
The WTO’s Council for Trade-Related Aspects of Intellectual Property Rights (TRIPS) :Under this license, the Lao pharmaceutical industry, as well as the pharmaceutical industry in similar countries(Bangladesh, Nepal, etc.), will be able to manufacture many drugs without patent authorization.
Reference:
《WTO members agree to extend drug patent exemption for poorest members》https://www.wto.org/english/news_e/news15_e/trip_06nov15_e.htm
《Product Patent Protection,the TRIPS LDC Exemption and the Bangladesh Pharmaceutical Industry》https://www.twn.my/title2/IPR/pdf/ipr17.pdf